Company profile
Shanghai BioGerm Medical Technology Co., Ltd., founded in March 2017, specializes in the R&D, production and sales of diagnostic reagents and equipment related to infectious pathogens. The Company also offers diversified services in terms of sequencing, scientific research and medical testing, providing customers with total solutions according to their specific needs.
Headquartered in Shanghai, the Company has an R&D center, a manufacturing center, a marketing center, a technical service center and a comprehensive management center, which cover a gross area of over 50,000m2. Besides, the Company has R&D sub-centers in Beijing and Guangzhou, a production-research integration base, and branches in many important cities/regions.
We survive on quality and innovate for further development. The Company has set up five major technology platforms - Fluorescent PCR Platform, Isothermal Nucleic Acid Amplification Platform, Immunity Platform, Instrument Manufacturing Platform and Sequencing Platform. As of March 2023, BioGerm has obtained certificate and medical device licenses from about 10 countries, including 28 in China, 68 in the EU CE, 3 in Saudi Arabia, 2 in Brazil, and 1 in Russia etc. The company is a high-tech enterprise in China and has won many government honors, including Shanghai "SRDI" Enterprise, Shanghai Patent Work Pilot Demonstration Unit, Shanghai 2022 Industry-Education Integration Enterprise, etc.
The Company has developed more than 500 types of reagents and instruments for nucleic acid testing and antigen/antibody rapid test on pathogens.
BioGerm upholds the core values of customer-first, innovation-driven, gratitude and altruism, is committed to serving more than 5,500 end users, winning wide acclaim from them.
Corporate Positioning
With a focus on accurate and rapid diagnosis of infectious pathogens, we are devoted to offering systemic solutions to various infections.
We aspire to create a material-intelligent manufacturing-diagnostic reagent-medical service industry chain for pathogen diagnosis.




Corporate Mission
Empowering Doctors, Enhance Life
To shorten patient’s visit time by 1 hour with BioGerm products
and services
Corporate Mission
Corporate Vision
To be a world-class expert in pathogen diagnosis
Corporate Vision
Values
Customer-first, innovation-driven, grateful and ready to help
Values

Development
AprilNovel Coronavirus (2019-nCoV) Antigen Detection Kit (Colloidal Gold) was granted the NMPA Registration Certificate
Novel Coronavirus (2019-nCoV) Antigen Detection Kit (Colloidal Gold) was granted CE Certification
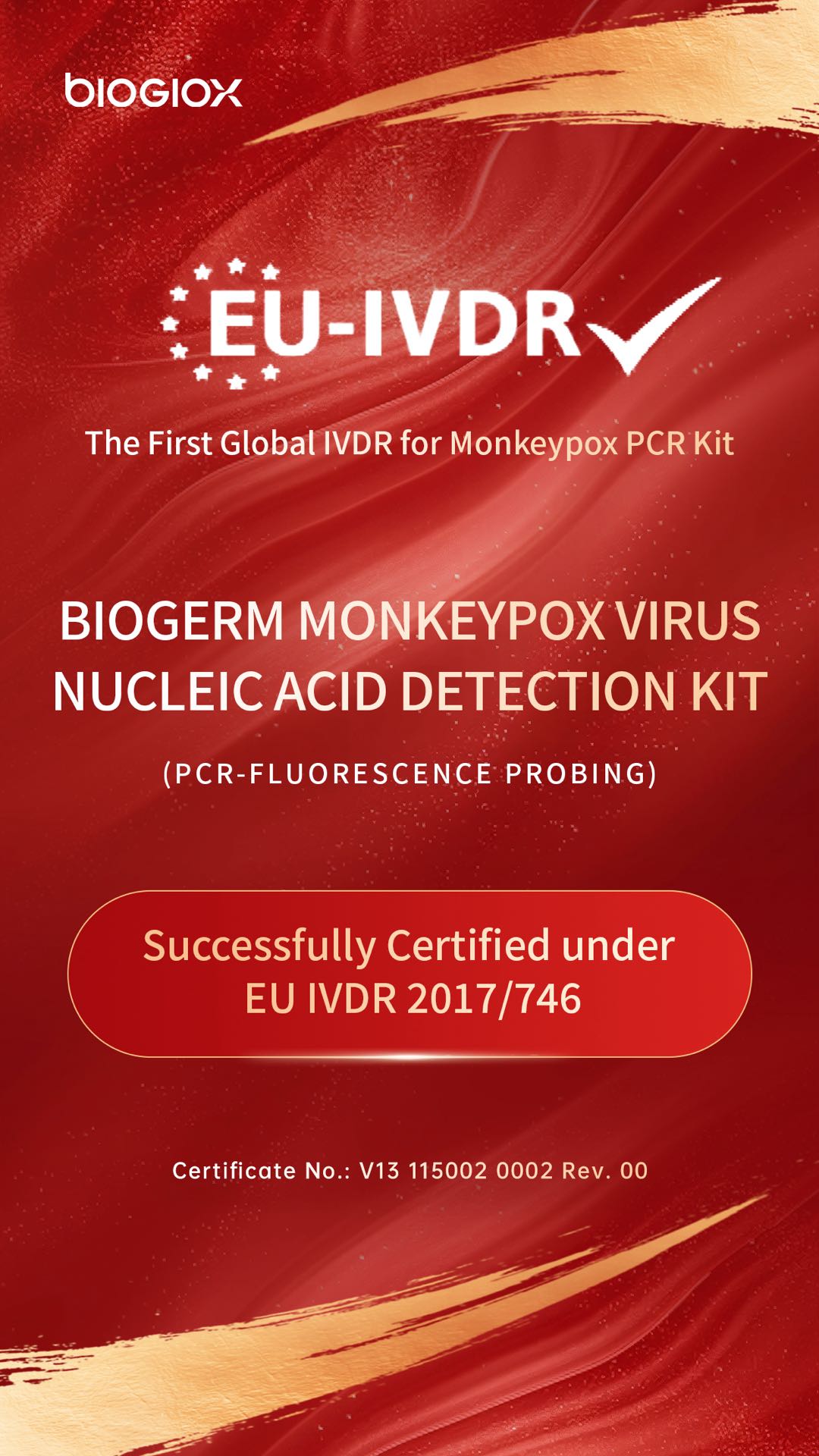
May21 varieties of viral nucleic acid detection kits were granted CE Certification
Monkeypox Virus Nucleic Acid Detection Kit was granted CE Certification
JanuaryBiogerm was awarded the 2020 Special Contribution Award of Shanghai Industrial Comprehensive Development Zone
JuneBiogerm came out among the best in the proportion of laboratories in the national inter-laboratory quality appraise
AugustBiogerm Six Respiratory Pathogens Nucleic Acid Detection Kit launched in the market
Biogerm Norovirus Nucleic Acid Detection Kit was approved for marketing
SeptemberThe Isothermal Nucleic Acid Amplifier was granted the NMPA Registration Certificate
Biogerm was awarded the first prize of Shanghai Science and Technology Progress Award
JanuaryThe Coronavirus Nucleic Acid Detection Kit passed the validation of CDC, was granted the NMPA Registration Certificate, and was included in the government emergency capacity requisition list of Shanghai Municipal Working Committee of Economy and Informatization
MarchThe review by Brazil National Health Surveillance Agency was completed
JuneThe review by Malaysia National Pharmaceutical Control Bureau was completed
JulyThe review by Singapore Health Sciences Authority was completed
The review by Thailand Food and Drug Administration was completed
The review by Philippine Food and Drug Administration was completed
JanuaryThe company was awarded the first batch of top 100 scientific and innovative enterprises of “Three Top 100 Enterprises” by Shanghai Fengxian District Government
AprilThe Second Biogerm Infectious Pathogen Detection Technology Seminar (Shanghai, China)
MayThe company was certified by ICAS ISO 9001/ISO 13485 management system
AprilBiogerm Tarich Virus Target Enrichment Kit for NGS sequencing was launched
JuneThe research and production platform of nucleic acid extraction using magnetic bead method was established
The third generation of virus sequencing technology platform was established
MarchShanghai Biogerm Medical Technology Co., Ltd. was founded
JuneThe research and development platform for pathogen fluorescent PCR kit was launched

2022

2021

2020

2019

2018













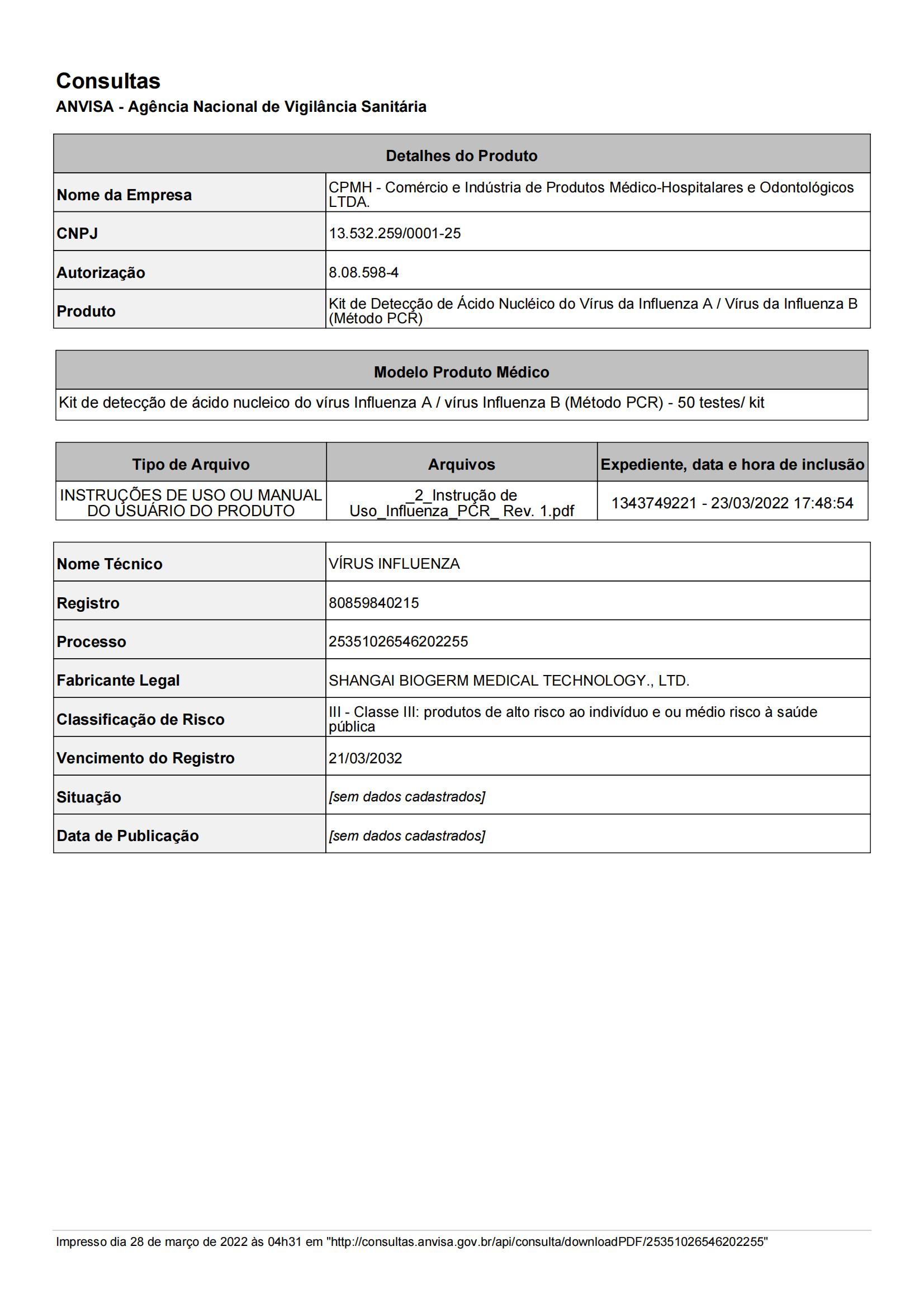
NucleicAcidDetectionKit(PCR-FluorescenceProbing)_00.jpg)

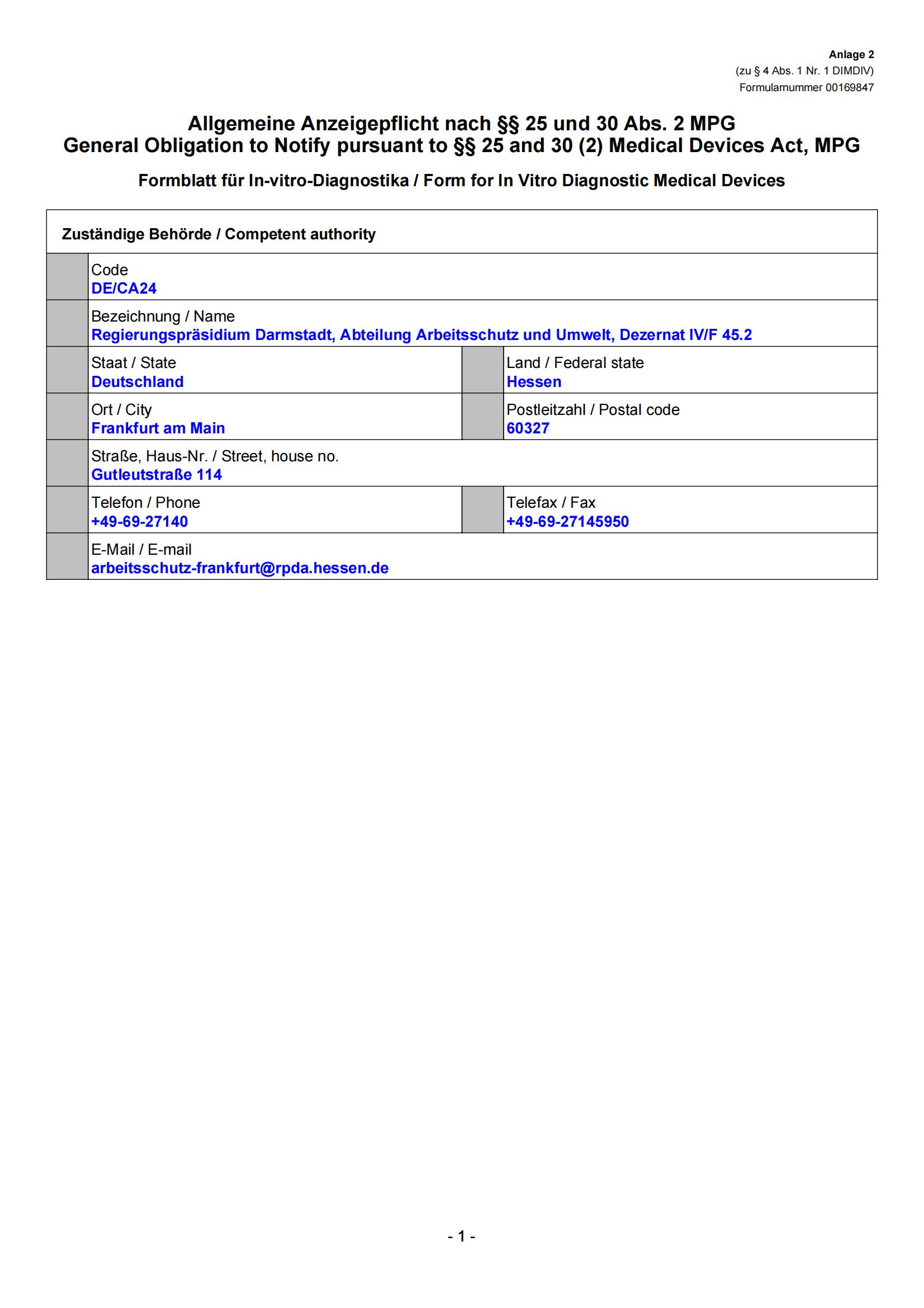
-EAR-Obelis-CE_00.jpg)

-1280757IVD-CE_00.jpg)

NucleicAcidDetectionKit.pdf_00.jpg)
NucleicAcidDetectionKit_00.jpg)
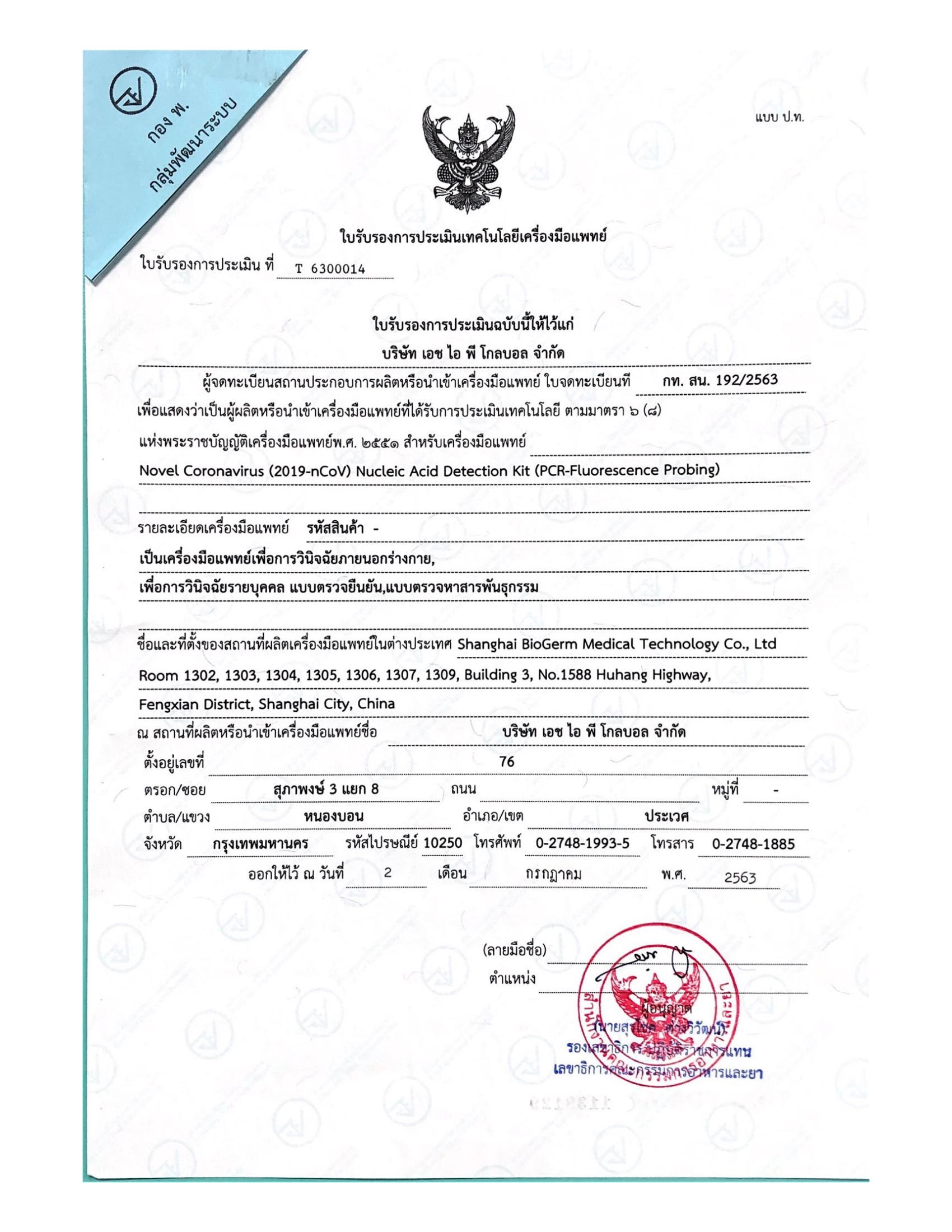
_00.jpg)
NucleicAcidDetectionKit-BioGerm-ClassDivddothers_signed_00.jpg)